20th NOVEMBER 2021 SYMPOSIUM AGENDA

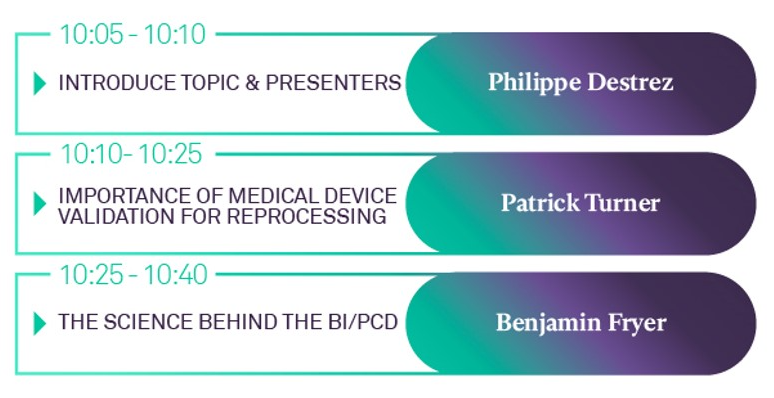
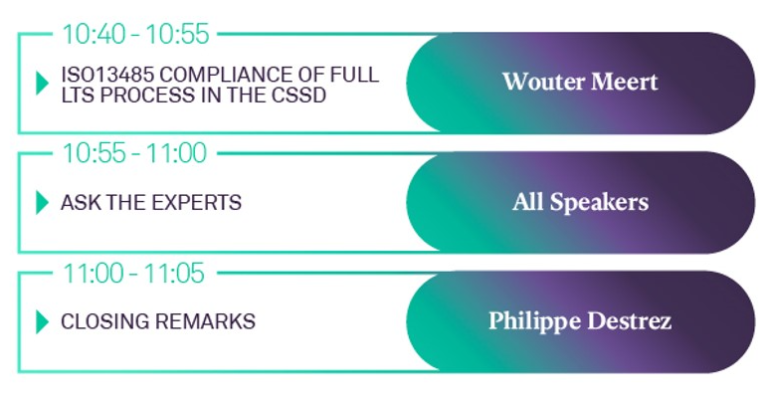
SPEAKERS


Over 30 years of experience in Hospital decontamination. BSc in Sterile Services Technology/management. MSc in Healthcare management. Authorized Person in Decontamination. Qualified Lead Auditor for EN ISO 13485. Decontamination Lead, Royal College of Surgeons of Ireland (RCSI) hospital group. Decontamination Manager & Lead, Beaumont Hospital Ireland.
Brief dive on the Importance of MD Validation to assure the right MD reprocessing. Check the different stakeholder’s responsibilities according to the guidelines (ISO14937/ISO17664/AAMI ST81) and the MDM IFU. Showcase associated benefits & value proposition in terms of Safety and Compliance.
“Comprehensive research on MD compatibility with its vital before medical devices are reprocessed for the first time. Monitoring over time is key to producing a quality product. Review the data and propose improvements were applicable.”


Ben Fryer is a Principal Scientist with Advanced Sterilization Products, where he is a leader in the Advanced Research and Early R&D groups. Ben is the technical lead in many of ASP’s products including the STERRAD™ 100NX System, STERRAD NX™ System and recently the STERRAD VELOCITY™ BI/PCD. Ben is a published author, holding more than 50 patents worldwide and has won several industry awards for medical device design excellence. Ben is also active on AAMI and is a US ISO representative for biological indicators.
What is a BI/PCD? New advances on the Monitoring explaining the fast BIs technology and focus on the PCD concept and associated benefits & value proposition in terms of Safety and Compliance
“A Biological Indicator BI) is used only demonstrate necessary conditions were met to achieve sterilization. A Process Challenge Device (PCD) is better than BI because assess process performance by providing a challenge equal or greater than most difficult items routinely processed”


Over 22 years of experience in Hospital decontamination. Process, project CSA Lead. Instrument management Lead. Board member VSZ (Flemish sterilization Society). Board member VVOV (Flemish society of operating room nurses). Member of the superior
health council ministry of Healthcare, infection prevention, development Belgian guidelines.
Teaching sterilization courses in various schools. Member Working group, ministry of Healthcare. Management training risk analyses, ISO 13485, MDR 2017-745, International auditor. Speaker at various congresses.
New advances in Monitoring & Validation explaining the importance of fast BI/PCD & IMS & Access for the process validation and associated benefits & value proposition in terms of Quality and Compliance (ISO 13485).
“Low temp sterilization by Hydrogen Peroxide became a standard in the reprocessing of Medical Devices. Take the opportunity to create a qualitative process in your CSSD based on the ISO 13485”.
TAKE HOME MESSAGES:
Here are the symposium’s assets
You can download the factsheet and see and listen to the symposium’s recording






