
Airway management devices are vital tools across various healthcare settings, including inpatient, outpatient, and emergency care. Proper use, maintenance, and reprocessing are critical to preventing healthcare-associated infections (HAIs) and enhancing patient outcomes.
This webinar explores common airway devices, their role in patient care, and how they can act as vectors for pathogenic microorganism transmission. Using scientific evidence, it examines reprocessing challenges, infection outbreaks linked to these devices, and strategies for safer, more sustainable practices. The ultimate goal is to safeguard both patient and healthcare worker safety while improving the efficacy of airway device management.
Airway devices are instruments used to access, examine, and treat parts of respiratory system – nasal cavity, larynx, and bronchi. These instruments are used in emergency, outpatient, and inpatient settings to ensure airway patency and ventilation of the lungs, facilitate intubation, anaesthesia, clinical investigations, and treatments of different degrees of invasivity. Ear, nose, throat (ENT) scopes, laryngoscopes and bronchoscopes are examples of airway devices ranging from simple rigid mechanical implements to complex flexible instruments containing fibre optics, electronics, and internal channels. Traditionally airway devices are considered semi-critical according to Spaulding classification as they are supposed to come in contact with mucosal epithelium of the airways but not with sterile body tissues. In today’s healthcare airway devices are often used in invasive procedures involving examination of deep bronchi, breach of blood vessels, biopsies, and minor surgical interventions.
As estimated by the FDA up to 500,000 bronchoscopies are performed annually in the USA while it is assumed bronchoscopes are utilized in a million or more procedures per year1. In the UK up to 2.8 million patients receive general anaesthesia and maybe subject to intubation every year. Number of nasal endoscopies done with the USA Medicare & Medicaid Services surpassed 500,000 in 2016 as annual procedure numbers increased by 9.3% over 15 years.
Such large numbers of procedures involving airways are inevitably leading to infection transmission and infection outbreaks. First reports of bronchoscope-related infection transmission were published in 1978 just as bronchoscopy was introduced in routine clinical practice. Since then, number of reports on infection or device contamination associated with reprocessed flexible bronchoscopes has been steadily growing and surpassed 200 in 2017.
Safe and efficient decontamination is the key factor in preventing infection transmission via airway devices. Single use bronchoscopes, ENT instruments and laryngoscopes as well as single use accessories are manufactured in controlled environment in line with international standards and terminally sterilized using Ethylene Oxide or irradiation. If stored and used in accordance with the manufacturers’ instructions risk of infection transmission via a single use device is extremely low. Reusable instruments are still widely used in airway procedures as they may be preferred by clinicians, are more environmentally friendly and are a cheaper option if compared with single use instruments. However, recent research suggests that reusable bronchoscopes can on average cost more per procedure when costs of treating hospital infections are taken into account10.
In theory reprocessing of a reusable medical device is straightforward process which renders the device safe for subsequent patient use. In real life situations proper cleaning, disinfection and sterilization are tampered with by multiple challenges. Compliance with reprocessing guidelines and good decontamination outcome relies on instruments being processed without delays by competent, educated, properly supervised staff who understand risks of infection transmission, decontamination processes supported by adequate resources, and devices themselves being regularly serviced and properly maintained. Staff shortages, insufficient resources, lack of training, lapses in instrument maintenance, careless attitude all create opportunities for microorganisms to survive decontamination by forming biofilms or by being protected by residual bioburden in case of inadequate or delayed cleaning. Several studies have shown that non-compliance with reprocessing guidelines may lead to endoscope-related health care-associated infections, but even compliant high level disinfection processes turned out inadequate in decontamination of flexible endoscopes resulting in infection transmission, injury and death.
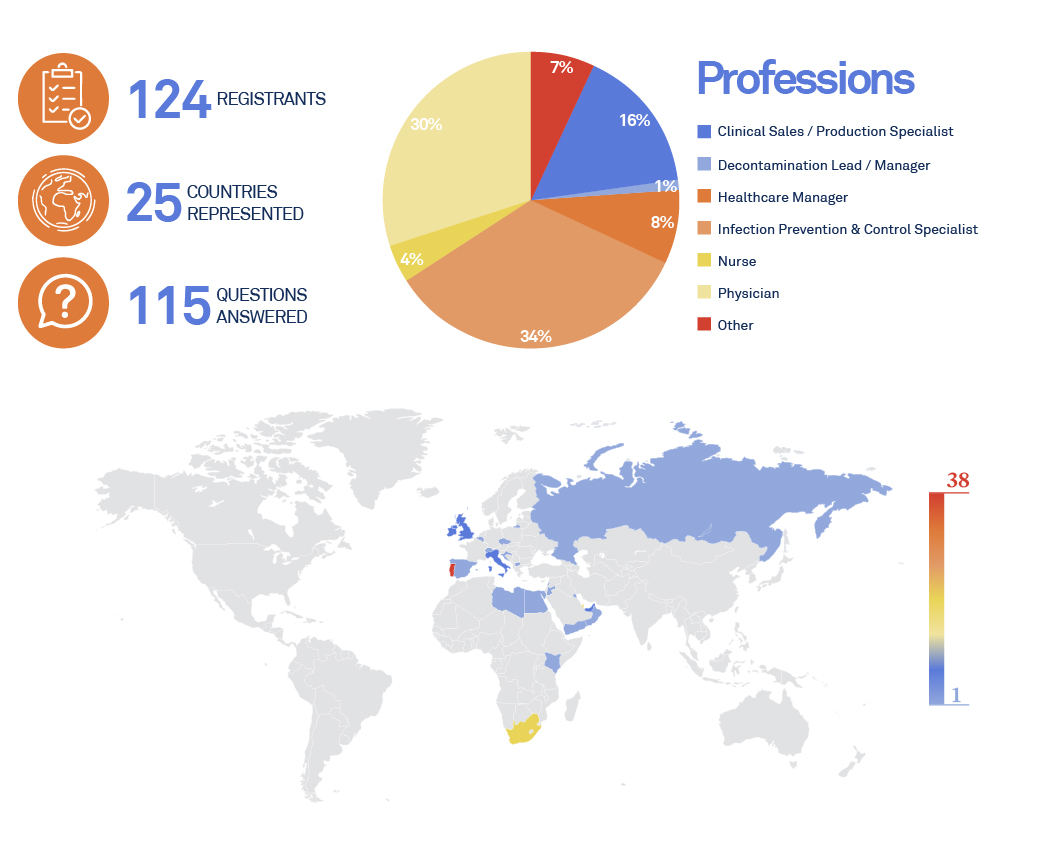
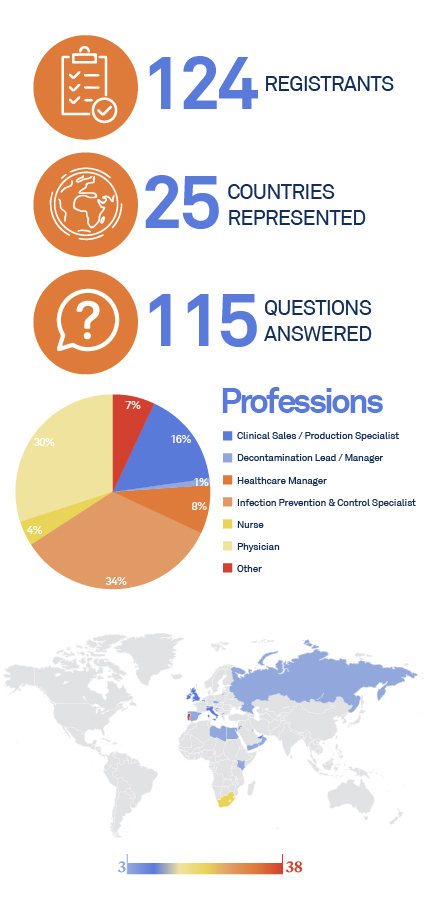
This is the third webinar dedicated to “Innovations In Airways Devices Reprocessing“, presented by ASP Continuous Education.
26th SEPTEMBER 2024


Mr. Olegs Tucs
- MSc Molecular and Cell Biology, BSc Decontamination Management.
- Associate Science Lecturer Technological University Dublin.
- Program Chair Higher Certificate in Medical Device Decontamination and BSc in Medical Device Decontamination Management. Technological University Dublin School of Chemical and BioPharmaceutical Sciences.
- Application Specialist with ASP Ireland.
- Experienced CSSD Manager, CSSD Technician.
- ESHRE Certified Senior Clinical Embryologist.
MESSAGES
-
Airway devices are diverse group of instruments used to examine and treat respiratory system issues, to ensure airway patency and facilitate intubation.
-
Airway devices are used on millions of vulnerable patients worldwide and contaminated instruments have been involved thousands of incidents and infection outbreaks.
-
High level disinfection is still an acceptable reprocessing option but as our understanding of pathogens increases, procedures become more invasive, and instruments get more complex it becomes substandard.
-
Biofilms can rapidly grow on instrument surface in presence of moisture and once established protect microorganisms from chemicals causing to disinfection failure
-
Prions are the hardest to destroy pathogens which are present in airways and can be potentially transmitted with inadequately decontaminated instruments
-
Success of decontamination cycle depends on correct performance of every reprocessing step and on employing most suitable decontamination technique


