- /
- Articles & Helpful Resources
- /
- Articles – FDA Safety Alert...
FDA Safety Alert about Infections Tied to Reprocessed Urological Endoscopes
On April 1, 2021, the US Food and Drug Administration (FDA) issued a safety alert in the form of a letter to health care providers regarding infections linked to reprocessed urological endoscopes. In this announcement, the FDA notes they have received numerous Medical Device Reports (MDRs) that describe patient infections post procedure or other possible contamination issues associated with reprocessing these devices.1
“We are very concerned about the three reported deaths—outside of the United States—associated with these infections, and we’re acting fast to communicate with health care providers and the public about what we know and what is still an emerging issue.”2 — Jeff Shuren, M.D., J.D., director of FDA’s Center for Devices and Radiological Health
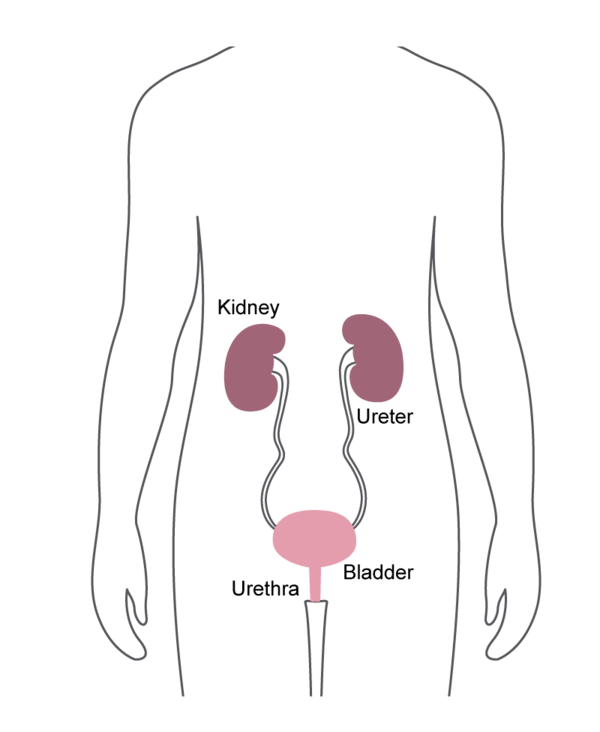
Cystoscopes
- Inserted into the bladder, via the urethra.
- Commonly used for removing bladder stones, or for treating bladder cancer.
Ureteroscopes
- Inserted through the bladder and into the ureter.
- Commonly used to find and sometimes treat kidney stones and other urinary tract problems.
Cystourethroscopes
- Inserted into the bladder, via the urethra.
- Commonly used to diagnose problems and to perform biopsies using surgical instruments passed through the tube.
Hundreds of flexible and semi-rigid urological endoscopes such as cystoscopes, uretheroscopes and cystoureteroscopes from leading device manufacturers such as Olympus, KARL STORZ, and Richard Wolf have been validated for STERRAD™ Systems. Visit the STERRAD™ Sterility Guide for a list of validated urological endoscopes.
An important note from the FDA:
The problems the FDA has identified with urological endoscopes and duodenoscopes may apply to similar devices. Therefore, the agency is also reviewing information on other types of endoscopes.2
What preceded this action?
Since 2017, the FDA has received over 450 Medical Device Reports (MDRs) which describe patient infections post procedure or other possible contamination issues associated with reprocessing these urological devices.1 Three of those adverse events included patient death; all three describe patients who developed Pseudomonas aeruginosa infections post procedure; one involved a cystoscope that did not pass a leak test.1
Where do endoscope reprocessing failures occur?
https://www.youtube.com/watch?v=Ri0yF20eM80
Check out this video on the hidden points of failure in endoscope reprocessing to learn more about where endoscope reprocessing failures typically occur.
FDA Recommendations (view full detail here):
- Carefully follow IFU reprocessing instructions, including precleaning, leak testing, cleaning, and either sterilization or disinfection + rinsing + drying
- Do not use damaged devices or those that fail leak test
- Develop schedules for routine inspection & maintenance
- Discuss benefits/risks associated with procedures involving reprocessed urological endoscopes with patients


What will the FDA do next?
Per the safety alert, “the potential causes and contributing factors associated with the reported infections or contamination issues are under review, including reprocessing methods, reprocessing instructions in the labeling, and device design.”1 They encourage health care providers to report any adverse events or suspected adverse events experienced with cystoscopes, ureteroscopes and cystourethroscopes devices using the MedWatch Voluntary Reporting Form.
ASP Recommendations
Given overwhelming evidence of potential points of failure in endoscope reprocessing via traditional high-level disinfection, ASP’s recommendation is simple:

This aligns with FDA recommendations. U.S. Food and Drug Administration guidance document Reprocessing Medical Devices in Health Care Settings: Validation Methods and Labeling. Guidance for Industry and Food and Drug Administration Staff states, “Users should be instructed to thoroughly clean [semi-critical devices] and then reprocess them by sterilization. If the device design does not permit sterilization (e.g., device materials cannot withstand sterilization), then high level disinfection should be used.”3
Sterilization compared to HLD
Disinfection and sterilization is necessary to ensure the safe reuse of medical devices. Sterilization is a process that destroys or eliminates all forms of microbial life whereas disinfection eliminates many or all pathogenic microorganisms, except bacterial spores.
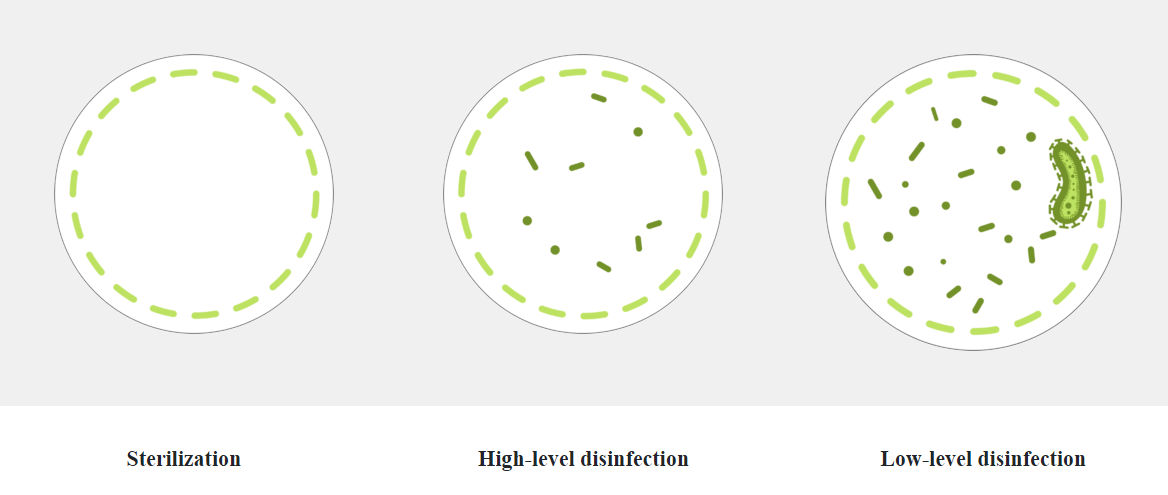
Remember, when it comes to endoscope reprocessing safety, ASP recommends:

References
- US Food and Drug Administration, Infections Associated with Reprocessed Urological Endoscopes – Letter to Health Care Providers: https://www.fda.gov/medical-devices/letters-health-care-providers/infections-associated-reprocessed-urological-endoscopes-letter-health-care-providers, Accessed April 5, 2021.
- US Food and Drug Administration. (2021, April 1). FDA is Investigating Reports of Infections Associated with Reprocessed Urological Endoscopes [Press release]. Retrieved from https://www.fda.gov/news-events/press-announcements/fda-investigating-reports-infections-associated-reprocessed-urological-endoscopes.
- U.S. Food and Drug Administration. (2015, March 17). Reprocessing Medical Devices in Health Care Settings: Validation Methods and Labeling. Guidance for Industry and Food and Drug Administration Staff.
