- /
- ASP Web Summit
- /
- Webinars Series #2 – Challenges...
- /
- Round Table – 6thWebinar –...
PANEL SPEAKERS
This is the sixth and last webinar dedicated to the “Challenges of Medical Device Reprocessing”, presented by ASP Continuous Education.
24th February 2022


Prof. Francesco Venneri | Clinical Risk Manager and Patient Safety Officer | Florence Healthcare System, Italy
Prof. Jon Otter | Honorary Senior Lecturer in HCAI AMR | Imperial College London
Mrs. Moya Alexander | Decontamination Lead | Imperial College Healthcare NHS Trust
Mrs. Soraia Pedroso | Clinical Nurse Specialist/ Lead Infection Prevention and Control | Nurse at Hospital Beatriz Ângelo, Lisbon
Eng. Jonathan Hart | Head of Technological Innovation and Health Technology Assessment | Campus Bio-Medico University Hospital, Rome


MAIN TOPICS
MEDICAL DEVICE REPROCESSING
FROM HISTORY TO CURRENT CONCEPTS OF GOOD PRACTICE
HEALTHCARE-ASSOCIATED INFECTIONS
RELATED WITH MEDICAL DEVICE REPROCESSING
ROBOTIC AND LAPAROSCOPIC SURGERY MD REPROCESSING:
PITFALLS AND OPPORTUNITIES FOR IMPROVED SAFETY
THE CHALLENGE OF ENDOSCOPIC REPROCESSING:
MOVING TOWARDS HIGHER STANDARDS
HEALTH TECHNOLOGY ASSESSMENT
FOR MD REPROCESSING IMPROVEMENT
TEASER
WEBINAR STATISTICS
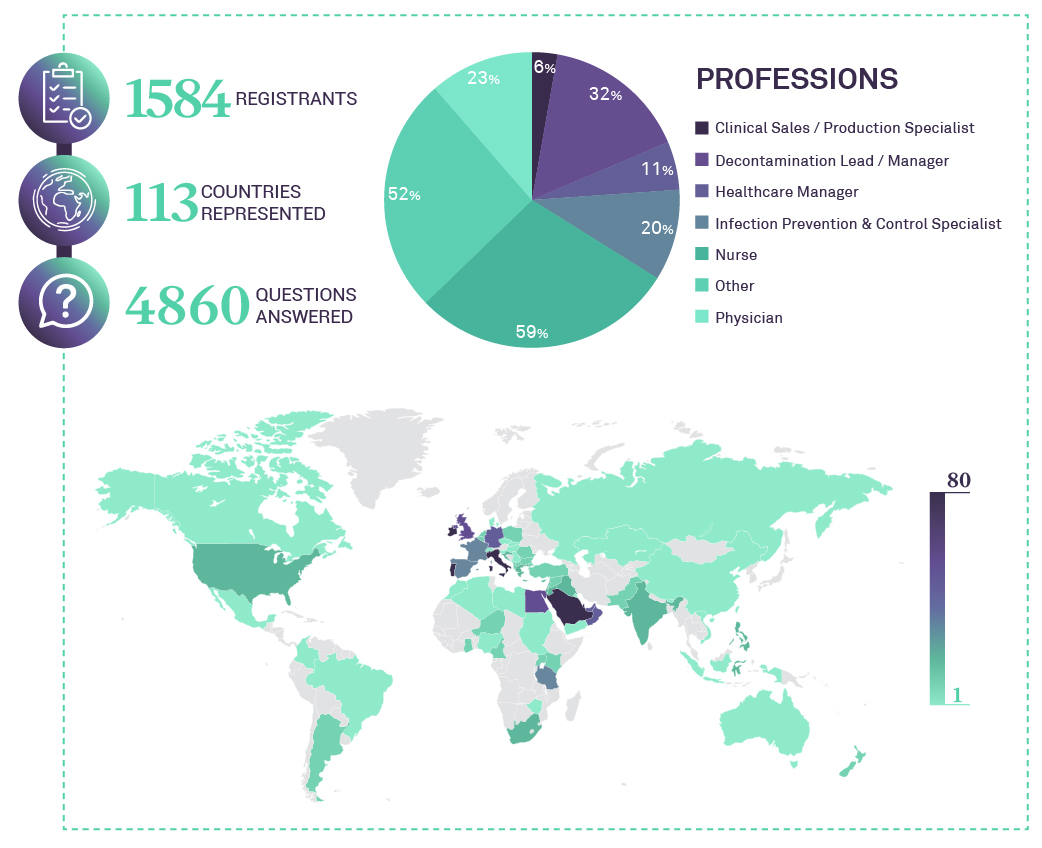
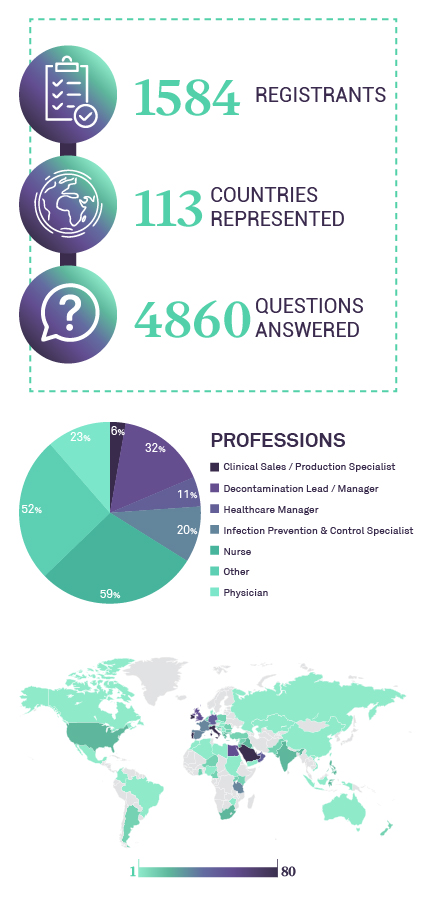
AUDIENCE FEEBACK
SPEAKER QUOTE
"According to the latest ECRI report, Device Cleaning, Disinfection, and Sterilization is on the top 10 of Patient Safety Concerns."
TAKE HOME
MESSAGES
MESSAGES
- Correct reprocessing steps and their knowledge are weapons against SSIs and assure patient trustworthiness in healthcare institutions.
- A look at medical device decontamination of the future: As medical devices become more complicated, the risks of inadequate decontamination are likely to grow; we need to embrace the latest systems to ensure the
safest possible decontamination. - There are challenges with minimal invasive surgeries and robotic instruments and they are extremely expensive.
- Quality assessment of the reprocessing process is mandatory and essential to ensure compliance with guidelines. ATP is a good marker for the cleaning
- HTA combines the appraisal of the technological, clinical, economic and organizational factors of reprocessing.



