AGENDA




General Announcement
“Championing Safety and Sustainability in Medical Device Reprocessing: Advancing Sterilization and Endoscopy Departments”
Despite the existence of comprehensive reprocessing guidelines and advances in the methods of reprocessing of Medical Device (MD), improper reprocessing of complex devices still contributes to a significant proportion of Healthcare-Acquired Infections (HAIs). 1 Of those, 18,3% of all HAIs in Europe are SSIs, according to the ECDC.2
Further, a 2020 ECRI report showed that Device Cleaning, Disinfection, and Sterilization is on the top 10 of Patient Safety Concerns.3
In recent years, new challenges have been associated with innovative surgical techniques, such as robotic and minimally invasive surgery, as well with modifications of endoscopic devices. The reprocessing of the devices used in these procedures is challenging and requires appropriate techniques. Complex devices coupled withinstances of inappropriate reprocessing practices have resulted in several outbreaks of infections; some were caused by multidrug-resistant bacteria related to endoscopic procedures, which highlights the importance of adequate reprocessing of the MD used in these techniques. 4,5
Improving the reprocessing of medical devices, particularly endoscopes, should be considered a major target for healthcare institutions to achieve in compliance with the highest standards of quality, efficacy, and safety. 6,7,8
At the same time, the application of the Circular Economy in the healthcare sector, which can be defined as a systems solution framework that tackles global challenges like climate change, biodiversity loss, waste, and pollution, must be regarded as an important framework to foster a better planet and an alternative way to attain economic growth and profits.9
New design strategies can be adopted to implement the Circular Economy in the healthcare sector, which depend on the value and the critical degree of the medical devices, resulting in lesser waste and longer durability of the equipment.10
Alternative reprocessing methods, namely low temperature instead of steam, could improve efficiency and result in better economic outcomes for a facility.11
SPEAKERS

MD, MS, MBA

Ivan S. Salgo, M.D., M.S., M.B.A. is Vice President, Chief Medical and Scientific Officer at Advanced Sterilization Products (ASP). He is keenly interested in providing a holistic picture on fortifying the need understanding and preventing Hospital Associated Infection and elevating the standard of care.
He obtained his M.D. degree in Perioperative Medicine and Surgical Critical Care from the Mount Sinai School of Medicine, his B.S. and M.S. degrees in Chemical Engineering from Columbia University, and his M.B.A. degree from the Massachusetts Institute of Technology Sloan School of Management. Dr. Salgo has 10 years of critical care experience and is a former faculty member at the University of Pennsylvania School of Medicine. His other accomplishments include 76 peer-reviewed publications and multiple grants, including NIH funded Surgical Research.
Healthcare Associated Infection & Antibiotic Resistance pose a major safety challenge to patients and hospital staff. This lecture will provide a review of what Sterilization Personnel should know about the state of HAIs in 2023 including the tactics organisms use to evade safety measures. These include the formation of biofilms and how bacteria evade antibiotic therapy. Armed with this information, the lecture will provide science-based recommendations for Sterilization Personnel on how they can safeguard patients, medical equipment, and themselves.

MSPH

Cori Ofstead is an epidemiologist with a Master of Science in Public Health degree and 30 years of experience in real-world research on infection prevention and device processing. Her studies have been published in AJIC, CHEST, Endoscopy International Open, Journal of Urology, and AAMI’s BI&T. She is a reviewer for several peer-reviewed journals and serves on AJIC’s editorial board and several standards-development committees.
Effective endoscope processing remains a challenge, especially as superbug infections linked to endoscopy become more common. In the first part of this session, an epidemiologist will present real-world evidence on endoscope processing effectiveness and discuss factors that contribute to the inadvertent continued use of damaged and/or contaminated devices from one patient to the next.

MSHA, MPH

Frank Daniels holds a Master of Science in Health Administration and a Master in Public Health degree and serves as the Director of High-Level Disinfection and Sterilization at Virginia Commonwealth University Health. In 2012, Frank centralized and founded the high-level disinfection program. He is a highly regarded expert in sterile processing quality management, and his studies have been published in AJIC.
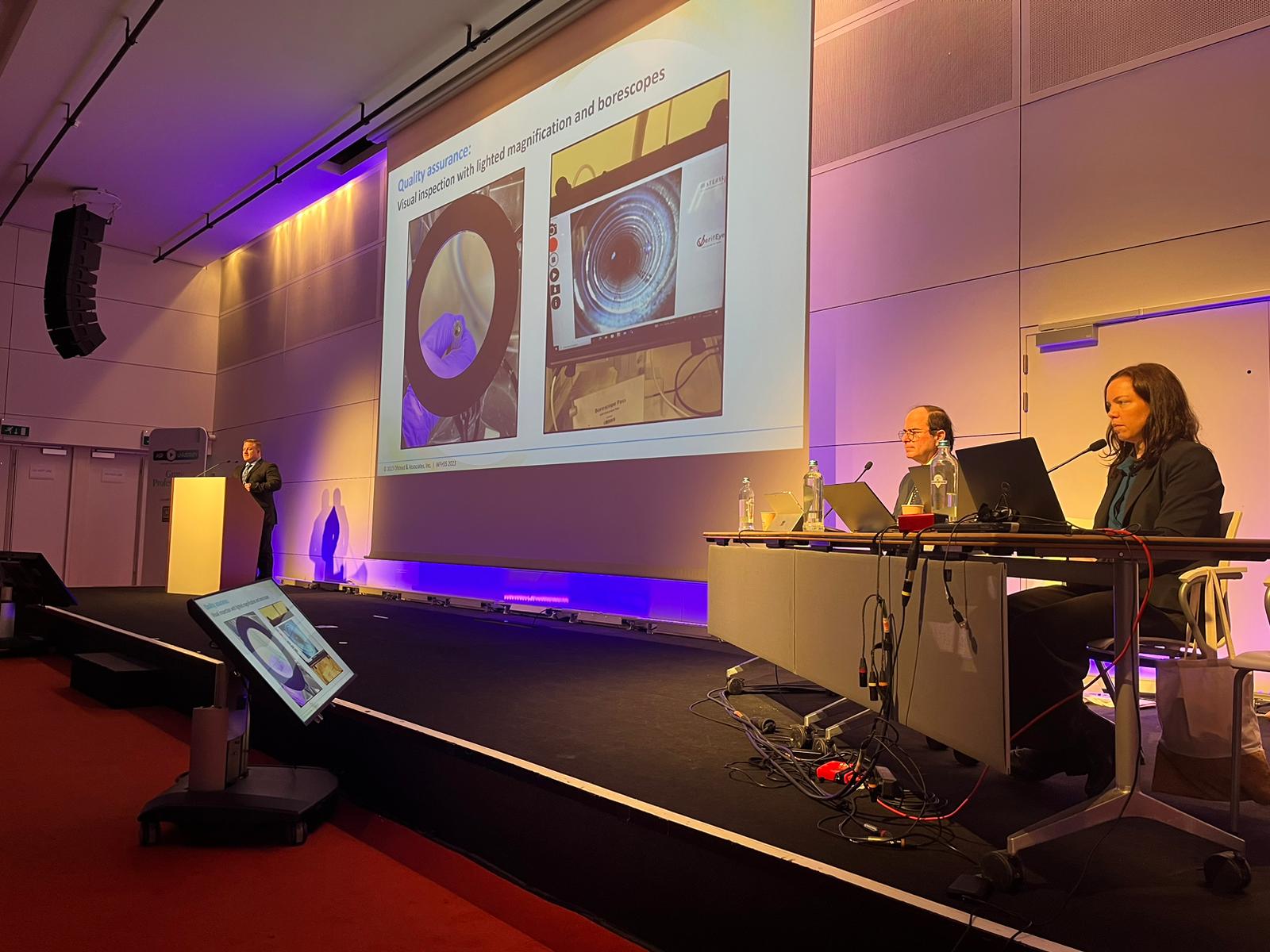
In the second part, a sterile processing expert will describe solutions that successfully addressed challenges associated with human factors, workflow, and quality assurance to improve occupational health and patient safety. He will also discuss his institution’s progress toward sterilization of flexible endoscopes.

MS, PhD

MSc in Health Policy Planning and Financing, Ph.D. in Health Economics
7 years of experience in Health Services Research
Expertise in Cost-Effectiveness Analysis & Health Economic Modeling
International experience – the UK and Australia
9 scientific and economic publications over the last 6 years
7 grants & awards on Health Economics for the past 2 years
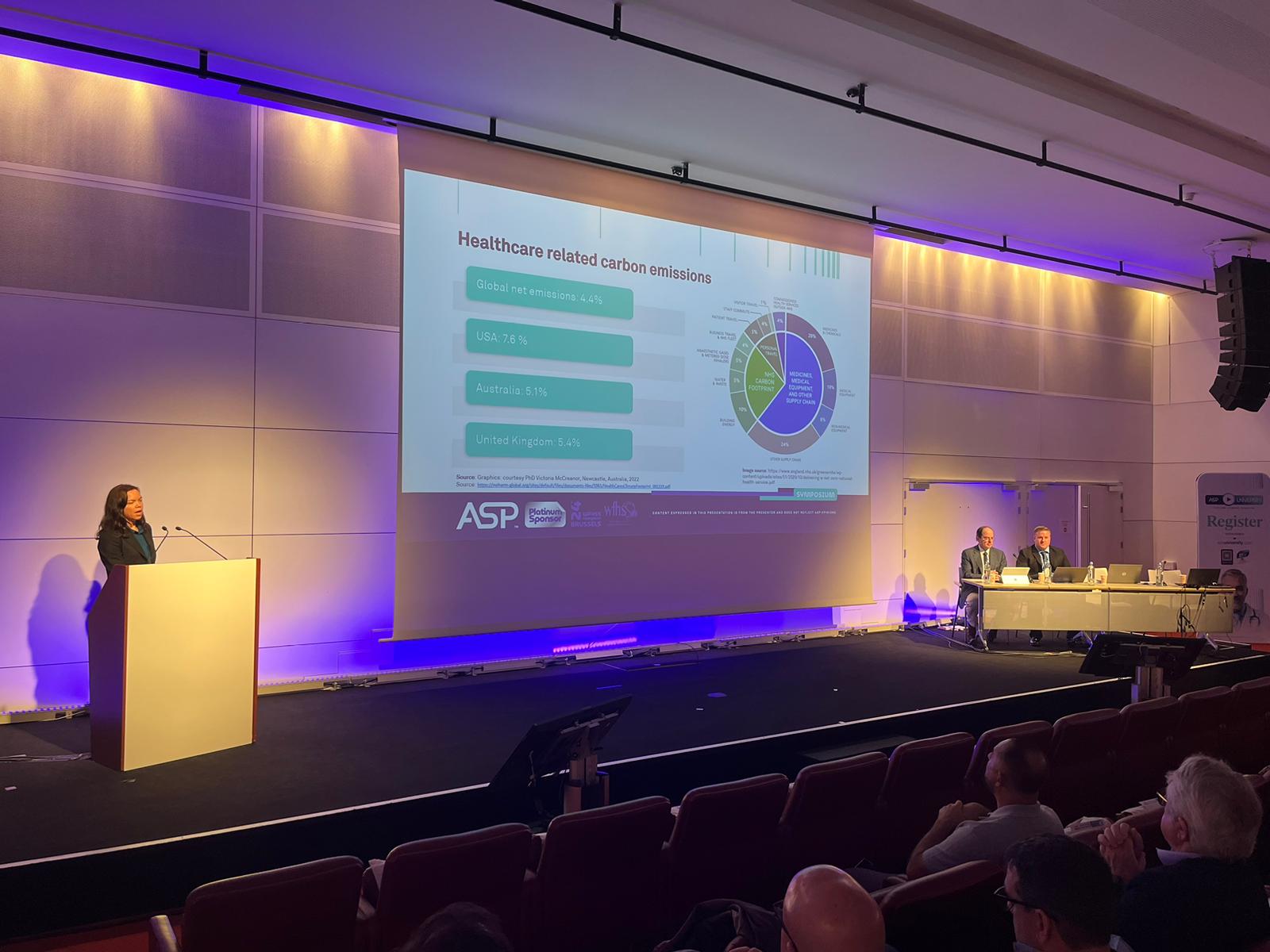
When considering which sterilizer is right for a particular setting, there is more to think about than the up-front costs of the sterilizer system itself. A comprehensive evaluation should assess longer-term costs and benefits of different options, including environmental considerations, in different settings. This presentation will cover an overview of climate considerations and carbon emissions healthcare, an introduction to health economic evaluation concepts, factors related to the sterilizer system, the instruments/devices being sterilizer and wrap up with how to use these concepts to evaluate different products.

TAKE HOME MESSAGES

- Global guidelines for the prevention of surgical site infection, second edition. Geneva: World Health Organization; 2018. (pag.27 3.1 Surgical site infection risk factors: epidemiology and burden worldwide). https://www.who.int/gpsc/ssi-prevention-guidelines/en/
- Prevalence of healthcare-associated infections, estimated incidence and composite antimicrobial resistance index in acute care hospitals and long-term care facilities: results from two European point prevalence surveys, 2016 to 2017 (Pag. 7, Table 3) https://www.eurosurveillance.org/content/10.2807/1560-7917.ES.2018.23.46.1800516
- Special Report Top 10 Patient Safety 2020 – Executive-Brief, ECRI, page 9, 5. Device Cleaning, Disinfection, and Sterilization. https://www.ecri.org/landing-top-10-patient-safety-concerns-2020
- Ofstead, C. L., Buro, B. L., Hopkins, K. M., Eiland, J. E., Wetzler, H. P., & Lichtenstein, D. R. (2020). Duodenoscope-associated infection prevention: A call for evidence-based decision making. Endoscopy international open, 8(12), E1769–E1781. https://doi.org/10.1055/a-1264-7173
- Scoping the problem – endoscopy associated infections – The Lancet Gastroenterology & Hepatology (www.thelancet.com/gastrohep Vol 3 July 2018)
- Dancer, S. J., Stewart, M., Coulombe, C., Gregori, A., & Virdi, M. (2012). Surgical site infections linked to contaminated surgical instruments. The Journal of hospital infection, 81(4), 231–238. https://doi.org/10.1016/j.jhin.2012.04.023. Journal Hosp Infection 2012 Aug;81(4):231-8.doi:10.1016/j.jhin.2012.04.023. Epub 2012 Jun 15.
- Southworth P. M. (2014). Infections and exposures: reported incidents associated with unsuccessful decontamination of reusable surgical instruments. The Journal of hospital infection, 88(3), 127–131. https://doi.org/10.1016/j.jhin.2014.08.007
- Tosh, P. K., Disbot, M., Duffy, J. M., Boom, M. L., Heseltine, G., Srinivasan, A., Gould, C. V., & Berríos[1]Torres, S. I. (2011). Outbreak of Pseudomonas aeruginosa surgical site infections after arthroscopic procedures: Texas, 2009. Infection control and hospital epidemiology, 32(12), 1179–1186. https://doi.org/10.1086/662712
- https://ellenmacarthurfoundation.org/topics/circular-economy-introduction/overview
- G.M. Kane, C.A. Bakker, A.R. Balkenende. Towards design strategies for circular medical products. Resources, Conservation and Recycling, Volume 135, 2018, Pages 38-47, https://doi.org/10.1016/j.resconrec.2017.07.030)
- McCreanor, V., & Graves, N. (2017). An economic analysis of the benefits of sterilizing medical instruments in low-temperature systems instead of steam. American journal of infection control, 45(7), 756–760. https://doi.org/10.1016/j.ajic.2017.02.026