The outbreaks with duodenoscopes identified in the beginning of this last decade have brought a big and real concern regarding the flexible endoscopes reprocessing since most of those instruments are complex to be proper cleaning. Besides the question is now if we should just high level disinfect them or sterilize them?
Outbreaks of healthcare associated infections caused by multi-drug resistant bacteria were associated with some endoscopic procedures. Endoscopes, in particular duodenoscopes and bronchoscopes, were pointed as the responsible for such situations, whose analysis revealed the inability of current reprocessing processes to effectively eliminate biological contamination. The combination of new design of duodenoscopes, increased invasiveness and the emergence of Gram negative resistant bacteria, such as carbapenem-resistant Enterobacterales made the perfect storm to these outbreaks. Worldwide market calls and corrective maintenance measures carried out by the manufacturers proved unable to correct the problem and to extinguish the risk of transmission related to the use of these devices.
Reprocessing of endoscopes is a complex process that requires adequate facilities, equipment and human factors. Design of facilities should focus on the possibility of having correct circuits of circulation of clean and contaminated devices. Adequate transport, cleaning, decontamination and storage should be considered in order to maximize safety. Manual or automated reprocessing can be done according to available resources. Teams of healthcare workers involved in the process should be appropriately selected, trained and maintained as constant as possible.
Reprocessing of endoscopes in this complex setting has become a major challenge for patient safety. Several methods are in use for this purpose, aiming the quality of high-level washing and disinfection processes of the endoscopes, namely ATP tests, microbiological studies for both endoscopes, reprocessing machines and validations of reprocessing cycles. In addition, traceability is a major component of endoscopic reprocessing in order to provide epidemiologic links whenever an endoscopic associated infection is suspected or confirmed.
Endoscopic procedures have become more and more aggressive, invading the mucosae and not simply touching them, as it was their primary purpose. The observed evolutions in the endoscopic procedures do contrast with the constancy of the Spaulding classification. According to this classification, these procedures do require higher levels of reprocessing in order to achieve appropriate safety. Given the possibility of incomplete microbiologic decontamination of endoscopes, sterilization can become the golden procedure for the reprocessing of endoscopic equipment, at least for specific devices and procedures. However, several limitations do exist for the implementation of this recommendation, namely related to the endoscopes, turnaround time, sterilization technologies, compatibility, the design of reprocessing units and the economic impact of such change. In this setting, the single-use endoscopes has also emerged as a new possibility to be taken into account.
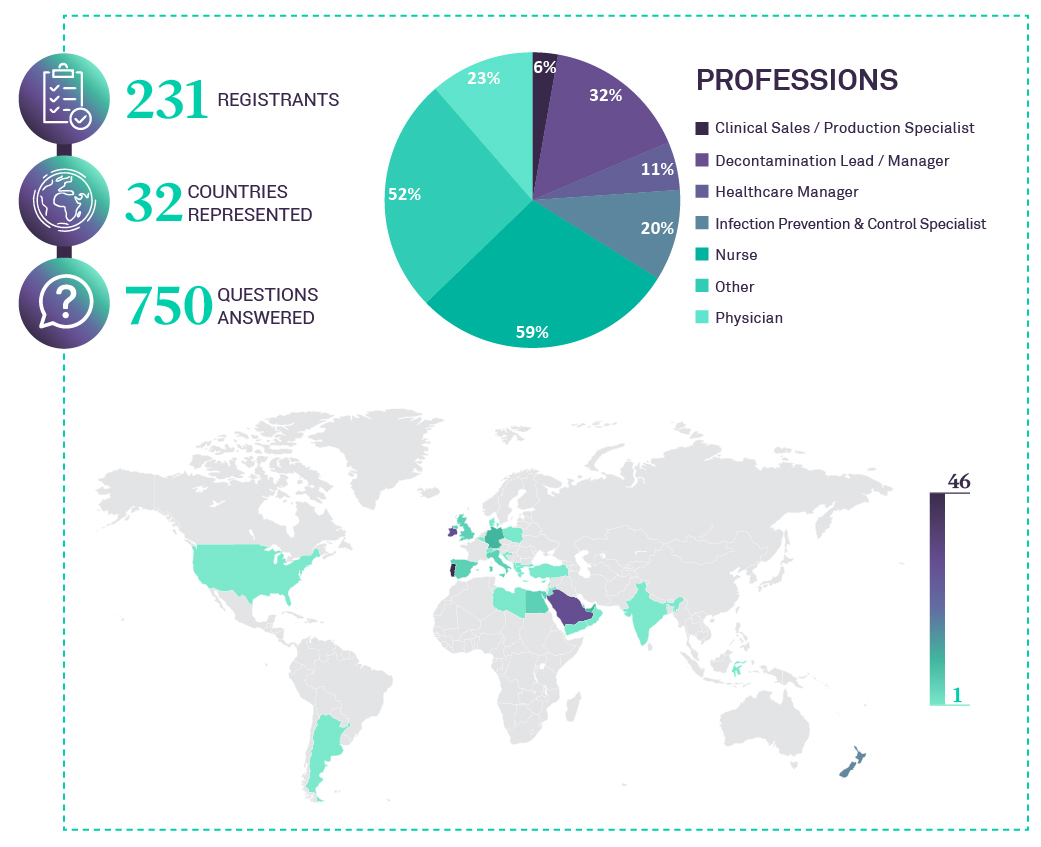
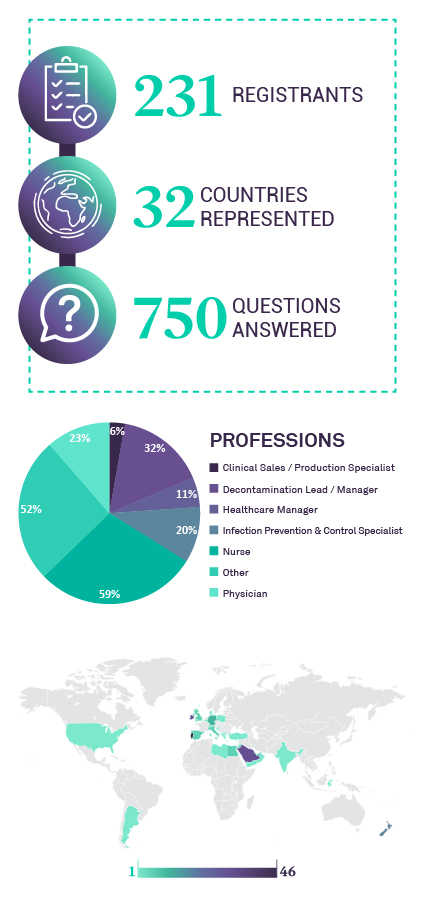
This is the forth webinar dedicated to “Challenges of Medical Device Reprocessing”, presented by ASP Continuous Education.
25h of November 2021


- Clinical nurse specialist/Lead Infection Prevention and Control Nurse
- 13 years as Infection Prevention & Control Nurse Practitioner (NP)
- Master’s of nursing (MSN) degree (2009)
- Postgraduate Course in Hospital Acquired Infections (2010)
- Finalist of the Best Practices in Health Portugal Award 10thedition (2016)
- Finalist of the Best Practices in Health Portugal Award 13thedition (2019)
MESSAGES
- Multidrug resistant organism (MDRO) and new genes exposed flaws in design, associating outbreaks to flaws and breaches in scope design rather than reprocessing steps compliance.
- Systematic and rigorous compliance with the endoscope reprocessing core elements is essential, but it does not meet the technical challenges of the complexity of the equipment. With all the major organizations that regulate good practice (CDC, ESGENA, FDA) encouraging the escalation of the safety levels of the reprocessing process.
- Quality assessment of the reprocessing process is mandatory and essential to ensure compliance with guidelines. Adenosine triphosphate (ATP) is a good marker for the cleaning process (validation of gross and organic removal) but can’t be used as a marker for the HL disinfection process or bacterial contamination, for that you should choose – microbiological culture sampling.
- MDRO and new genes exposed flaws in design, associating outbreaks to flaws and breaches in scope design rather than reprocessing steps compliance.
- There is no evidence that double HLD or HLD+Ethylene Oxide Sterilization (ETO) is better than single HLD cycle (access by ATP and microbiological sampling).
- But ETO is identified as the intervention that ended at least 3 published outbreaks.
- ETO is unlikely to be the answer to all problems…12 hours cycles, risk of toxicity and scarce equipment’s.
- Hydrogen Peroxide (HP) sterilization could be a solution for the flexible scopes sterilization but may not be readily available for all the gastroenterology endoscopes.
- Disposable scopes could resolve the infection control issues, but at an unbearable environmental and economic cost.
- Low temperature sterilization (LTS) seems to be the path to go, but industry must make combined efforts, new prototypes must address reprocessing needs – it´s not good if It´s not clean.



